Sorbitol

Sorbitol
Sorbitol - Premium Food-Grade Sweetener & Humectant
THE Novochem Group presents pharmaceutical-grade Sorbitol (E420), a versatile polyol sweetener widely used in food, pharmaceutical, and cosmetic industries. As a global leader in food additives, we manufacture Sorbitol under strict GMP conditions to ensure consistent quality across all batches. Our high-purity Sorbitol meets stringent international quality standards including USP, BP, EP, and FCC specifications, making it suitable for the most demanding applications in sensitive formulations.
Sorbitol occurs naturally in fruits like apples and pears, but commercial production involves the catalytic hydrogenation of glucose. This process yields a product with about 60% the sweetness of sucrose but with only 2.6 calories per gram. The Novochem advantage lies in our proprietary purification technology that removes residual sugars and byproducts, resulting in superior stability and performance characteristics compared to standard industry offerings.
Chemical Composition
IUPAC Name: (2S,3R,4R,5R)-Hexane-1,2,3,4,5,6-hexol
Chemical Formula: C6H14O6
CAS Number: 50-70-4
Molecular Weight: 182.17 g/mol
Structure: Sugar alcohol derived from glucose by hydrogenation
Chemically, Sorbitol is a hexitol with six hydroxyl groups that give it excellent water solubility and humectant properties. The spatial arrangement of these hydroxyl groups creates a stable molecular configuration that resists crystallization better than other polyols. Our manufacturing process controls the stereochemistry to ensure optimal functional performance, particularly important for pharmaceutical applications where consistency is critical.
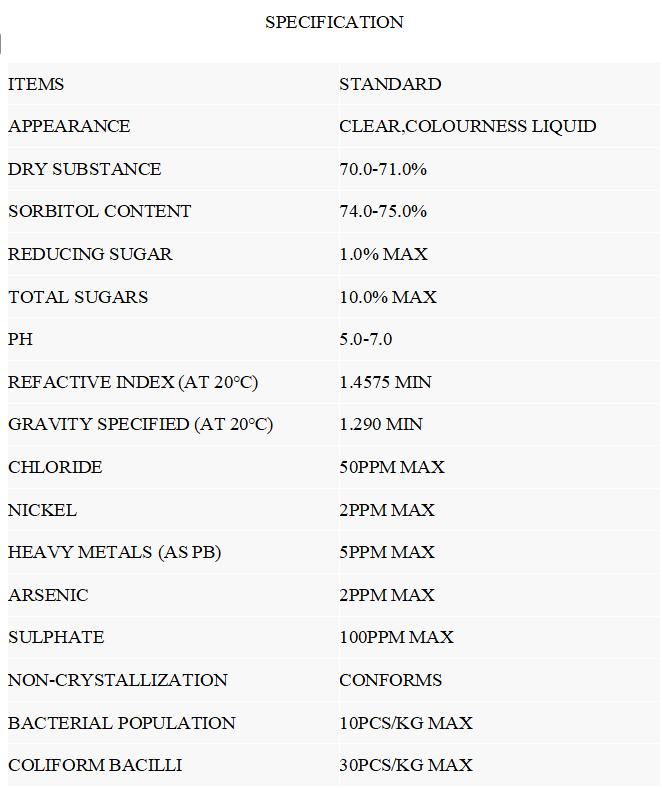
Certificate of Analysis (COA)
Functional Properties & Applications
Food Industry Applications
Sorbitol serves multiple functional roles in food systems beyond sweetness:
Sugar-free confectionery: Primary bulking agent in chewing gum (25-35% typical usage), hard candies, and chocolates where it provides body and mouthfeel without promoting dental caries
Baked goods: Moisture retention agent extending shelf-life by 30-50% in cakes and cookies at 5-15% inclusion rate
Diabetic foods: Low glycemic index (9) makes it suitable for sugar-reduced formulations with only 50% absorption rate in small intestine
Frozen desserts: Anti-crystallization agent in ice cream (typically 3-8%) improving texture and scoopability
Flavor systems: Carrier for spray-dried flavors and stabilizer for volatile aromatics
Meat products: Humectant in jerky and processed meats at 1-3% levels
Pharmaceutical Industry Uses
In drug formulations, Sorbitol provides multiple technical benefits:
Tablet excipient: Direct compression filler-binder with excellent compactibility (15-90% w/w common)
Syrups: Vehicle for pediatric formulations (30-70% in oral solutions)
Laxatives: Osmotic agent in constipation remedies (10-30g doses)
Chewable tablets: Provides pleasant cooling effect and masks bitter APIs
Lyophilization: Bulking agent for freeze-dried injectables
Toothpaste: Humectant in gel formulations (10-25%) preventing hardening
Cosmetic Formulations
Personal care applications leverage Sorbitol's unique properties:
Skin care: Moisturizer in creams/lotions (2-10%) with water-binding capacity of 350g/100g
Hair care: Plasticizer in styling products improving flexibility
Oral care: Synergistic humectant in toothpaste with glycerin
Color cosmetics: Binder in pressed powders and stabilizer for emulsions
Shaving products: Lubricant in gels reducing razor drag
In all applications, our Sorbitol provides superior batch-to-batch consistency with minimal variation in key functional parameters. Technical support available for formulation optimization and troubleshooting.
Storage & Handling Guidelines
Proper handling ensures optimal performance and shelf life:
Storage conditions: Original packaging in cool, dry warehouse (15-25°C ideal) with palletized storage recommended
Humidity control: Maintain relative humidity below 65% to prevent caking
Compatibility: Keep away from strong oxidizing agents - separate from peroxides, chlorates, and nitrates
Shelf life: 24 months from production date when stored properly in unopened containers
Handling: Use appropriate PPE (NIOSH-approved dust mask, gloves, eye protection) when handling large quantities
First-in-first-out: Rotate stock to ensure freshness
Re-packaging: Use stainless steel or food-grade plastic containers if transferring
Safety Information: Generally Recognized as Safe (GRAS) by FDA. May cause mild laxative effect if consumed in quantities exceeding 20-50g/day depending on individual tolerance. Not classified as hazardous under OSHA Hazard Communication Standard. MSDS available for download on our website with full handling precautions and emergency procedures.
Available Packaging Options
Flexible packaging solutions to match your production needs:
Small quantities: 25 kg multi-layer paper bags with food-grade PE liner (palletized 40 bags/pallet)
Bulk shipments: 500 kg and 1000 kg bulk bags (FIBC) with discharge spouts
Pharmaceutical grade: 20 kg double-lined bags with tamper evidence
Custom options: Smaller retail-ready packages, super sacks with liners, or dedicated silo trucks for large users
All packaging meets international transportation regulations including IMDG, IATA, and ADR. Custom labeling available with your specifications including barcodes, QR codes, and regulatory statements. Just-in-time delivery programs available for high-volume customers.
Regulatory Compliance
Our Sorbitol meets global regulatory requirements for food and pharmaceutical use:
United States: FDA 21 CFR 184.1835 (GRAS), USP-NF monograph compliant
European Union: Approved under EU Directive 2008/100/EC as food additive E420
International: JECFA Specifications, Codex Alimentarius Standard
Asia: Complies with China GB 1886.187, India FSSAI standards
Certifications: Kosher (OU), Halal, Non-GMO Project Verified
GMP: Manufactured in ISO 9001:2015 certified facilities with HACCP programs
We maintain current regulatory intelligence across all major markets and can provide supporting documentation for product registrations including Letters of Guarantee, Certificate of Conformance, and Country-of-Origin certificates. Our regulatory affairs team assists with novel food applications and dossier preparation for new market entries.